

Description
Y-shape PEG NHS Ester with superior quality specification of ≥95% Substitution.
Y-shape PEG NHS from JenKem Technology is a Succinimidyl Carboxymethyl Ester branched 2ARM PEG, reactive towards the amino group of lysine(s) on proteins or other biologics. Amine PEGylation with Y-shape PEG NHS can be completed in less than 1hr at pH 7-8. JenKem proprietary Y-shape PEGs are more selective, due to their sterically bulky structure. Please review the application note for this product for additional instructions for amine PEGylation with Y-NHS-40K.
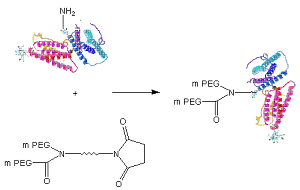
Schematic of Protein PEGylation with JenKem® Y-PEG-NHS
JenKem Technology offers Y-shape PEG NHS with MW 40000, in 1g and 10g packing sizes. JenKem Technology provides repackaging services for an additional fee, please contact us if you require a different package size than our catalog selection.
Different MW of Y-shape PEG NHS products may be available by custom synthesis, please email us at tech@jenkemusa.com for details on custom PEGs.
Bulk PEGs and GMP grade PEGs are made-to-order. Please contact us for bulk pricing.
Application of Y-NHS-40K for PEGylation:
| Drug Molecule or Other Entity PEGylated with Y-NHS-40K | References |
| Calcium phosphate nanoparticles | 7 |
| Cp40 | 4 |
| DNA aptamer (SOMAmer) | 2 |
| G-CSF | 15 |
| Gentamicin | 14 |
| IFN-α2a | 16 |
| IFN-α2b | 17 |
| LIF receptor antagonist (LA) | 11 |
| L-RNA (Spiegelmer) | 5 |
| L-RNA (Spiegelmer) | 8 |
| rhGH | 18 |
| TNF-α | 9, 10 |
Click here to download the MSDS
References:
1. AlQahtani, A.D., et al., Production of “biobetter” glucarpidase variants to improve drug detoxification and antibody directed enzyme prodrug therapy for cancer treatment, European Journal of Pharmaceutical Sciences, 2019, 127, P. 79-91.
2. Haruta, K., et al., A Novel PEGylation Method for Improving the Pharmacokinetic Properties of Anti-Interleukin-17A RNA Aptamers, Nucleic acid therapeutics, 2017, 27(1):36-44.
3. Guo, L., et al., Application Instructions for Y-NHS-40K for Amine PEGylation, click link.
4. Masao, H., et al., Chemically Modified Interleukin-6 Aptamer Inhibits Development of Collagen-Induced Arthritis in Cynomolgus Monkeys, Nucleic Acid Therapeutics, 2015.
5. Winship, A.L., Interleukin-11 alters placentation and causes preeclampsia features in mice, Proc Natl Acad Sci U S A., 2015, 112(52):15928-33.
6. , A.M., et al., Peptide inhibitors of C3 activation as a novel strategy of complement inhibition for the treatment of paroxysmal nocturnal hemoglobinuria, Blood Mar, 2014, 123 (13) 2094-2101.
7. Roccaro, Aldo M. et al., SDF-1 Inhibition Targets the Bone Marrow Niche for Cancer Therapy, Cell Reports, 2014, 9 (1), p: 118 – 128.
8. Stefan, N., et al., Novel Prodrug-Like Fusion Toxin with Protease-Sensitive Bioorthogonal PEGylation for Tumor Targeting, Bioconjugate chemistry, 2014, 25.12: 2144-2156.
9. Ashokan, A., et al., Multifunctional calcium phosphate nano-contrast agent for combined nuclear, magnetic and near-infrared in vivo imaging. Biomaterials, 2013, 34(29): p. 7143-7157.
10. Khan, M.A., et al., Targeting complement component 5a promotes vascular integrity and limits airway remodeling, PNAS, 2013, 110(15) p:6061-6066.
11. Dai, C.Y., et al., Preparation and evaluation of a new releasable PEGylated tumor necrosis factor-α (TNF-α) conjugate for therapeutic application, Science China Life Sciences, 2013, 56.1 : 51-58.
12. Dai, C.Y., et al., Linkage with cathepsin B-sensitive dipeptide promotes the in vitro and in vivo anticancer activity of PEGylated tumor necrosis factor-alpha (TNF-α) against murine fibrosarcoma, Science China Life Sci, 2011, 54(2): 128–138.
13. Menkhorst, E., et al., Vaginally Administered PEGylated LIF Antagonist Blocked Embryo Implantation and Eliminated NonTarget Effects on Bone in Mice, PLoS ONE, 2011, 6 (5) e19665.
14. Cai, Y., et al., Separation of exenatide analogue mono-PEGylated with 40 kDA polyethylene glycol by cation exchange chromatography, Journal of Chromatography A, 2011, 1218:39, P. 6953-6960.
15. Wang, Y-J., et al., PEGylation markedly enhances the in vivo potency of recombinant human non-glycosylated erythropoietin: A comparison with glycosylated erythropoietin, Journal of Controlled Release, 2010, 145:3, p. 306-313.
16. Marcus, Y., et al., Turning Low-Molecular-Weight Drugs into Prolonged Acting Prodrugs by Reversible Pegylation: A Study with Gentamicin, Journal of Medicinal Chemistry, 2008, 51 (14), 4300-4305.
17. Wang, S., et al., Y-type polyethylene glycol modified G-CSF and preparation method and use thereof. Patent CN200780051378, 2007.
18. Zhou, W., et al., Interferon alpha 2a modified by polyethylene glycol, its synthesis process and application. Patent CN200780050541, 2007.
19. Zhou, W., et al., Interferon alpha 2b modified by polyethylene glycol, its synthesis process and application. Patent CN200780050542, 2007.
20. Zhou, W., et al., Double-stranded polyethylene glycol modified growth hormone, preparation method and application thereof. Patent CN200880009718, 2008.
Founded in 2001 by experts in PEG synthesis and PEGylation, JenKem Technology specializes exclusively in the development and manufacturing of high quality polyethylene glycol (PEG) products and derivatives, and related custom synthesis and PEGylation services. JenKem Technology is ISO 9001 and ISO 13485 certified, and adheres to ICH Q7A guidelines for GMP manufacture. The production of JenKem® PEGs is back-integrated to in-house polymerization from ethylene oxide, enabling facile traceability for regulated customers. JenKem Technology caters to the PEGylation needs of the pharmaceutical, biotechnology, medical device and diagnostics, and emerging chemical specialty markets, from laboratory scale through large commercial scale.
JenKem Technology由PEG合成和PEG化的专家成立于2001年,专门从事高纯度,低多分散性的聚乙二醇(PEG)衍生物,PEG共聚物,单分散PEG,定制PEG衍生物合成和PEG化服务。通过获得本体环氧乙烷以及内部所有聚合和衍生化的性能来支持特定的强度。
JenKem Technology从研发到GMP商业批量提供全球PEG衍生物,用于临床前,临床试验以及用于制药,生物技术,医疗设备和诊断的商业产品。













