

Description
Y-shapePEGNHSEster withsuperiorqualityspecificationof ≥95%Substitution.
Y-shapePEGNHS fromJenKemTechnologyisaSuccinimidylCarboxymethylEsterbranched2ARMPEG,reactivetowardstheaminogroupoflysine(s)onproteinsorotherBIOLOGics.AminePEGylationwithY-shapePEGNHScanbecompletedinlessthan1hratpH7-8.JenKemproprietaryY-shapePEGsaremoreselective,duetotheirstericallybulkystructure.PleasereviewtheapplicationnoteforthisproductforadditionalinstructionsforaminePEGylationwithY-NHS-40K.
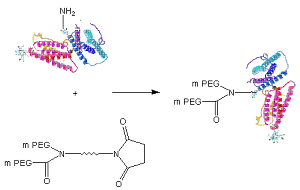
SchematicofProteinPEGylationwithJenKem®Y-PEG-NHS
JenKemTechnologyoffers Y-shapePEGNHS withMW40000,in1gand10gpackingsizes. JenKemTechnology providesrepackagingservicesforanadditionalfee,pleasecontactusifyourequireadifferentpackagesizethanourcatalogselection.
DifferentMWof Y-shapePEGNHS productsmaybeavailablebycustomsynthesis,pleaseemailus attech@jenkemusa.comfordetailsoncustomPEGs.
BulkPEGsandGMPgradePEGsaremade-to-order.Please contactus forbulkpricing.
ApplicationofY-NHS-40KforPEGylation:
| DrugMoleculeorOtherEntityPEGylatedwithY-NHS-40K | References |
| Calciumphosphatenanoparticles | 7 |
| Cp40 | 4 |
| DNAaptamer(SOMAmer) | 2 |
| G-CSF | 15 |
| Gentamicin | 14 |
| IFN-α2a | 16 |
| IFN-α2b | 17 |
| LIFreceptorantagoNIST(LA) | 11 |
| L-RNA(Spiegelmer) | 5 |
| L-RNA(Spiegelmer) | 8 |
| rhGH | 18 |
| TNF-α | 9,10 |
ClickheretodownloadtheMSDS
References:
1.AlQahtani,A.D.,etal.,Productionof“biobetter”glucarpidasevariantstoimprovedrugdetoxificationandantibodydirectedenzymeprodrugtherapyforcancertreatment,EuropeanJournalofPharmaceuticalSciences,2019,127,P.79-91.
2.Haruta,K.,etal.,ANovelPEGylationMethodforImprovingthePharmacokineticPropertiesofAnti-Interleukin-17ARNAAptamers,Nucleicacidtherapeutics,2017,27(1):36-44.
3.Guo,L.,etal.,ApplicationInstructionsforY-NHS-40KforAminePEGylation,click link.
4.Masao,H.,etal.,ChemicallyModifiedInterleukin-6AptamerInhibitsDevelopmentofCollagen-InducedArthritisinCynomolgusMonkeys,NucleicAcidTherapeutics,2015.
5. Winship,A.L.,Interleukin-11altersplacentationandcausespreeclampsiafeaturesinmice,ProcNatlAcadSciUSA.,2015,112(52):15928-33.
6. ,A.M.,etal., PeptideinhibitorsofC3activationasanovelstrategyofcomplementinhibitionforthetreatmentofparoxysmalnocturnalhemoglobinuria, BloodMar,2014,123(13)2094-2101.
7.Roccaro,Aldo M.etal., SDF-1InhibitionTargetstheBoneMarrowNicheforCancerTherapy, CellReports,2014,9(1),p:118–128.
8. Stefan,N.,etal.,NovelProdrug-LikeFusionToxinwithProtease-SensitiveBioorthogonalPEGylationforTumorTargeting,Bioconjugatechemistry, 2014,25.12:2144-2156.
9.Ashokan,A.,etal.,Multifunctionalcalciumphosphatenano-contrastagentforcombinednuclear,magneticandnear-infraredin vivoimaging.Biomaterials,2013,34(29):p.7143-7157.
10.Khan,M.A.,etal., Targetingcomplementcomponent5apromotesvascularintegrityandlimitsairwayremodeling,PNAS,2013,110(15)p:6061-6066.
11. Dai,C.Y., etal.,PreparationandevaluationofanewreleasablePEGylatedtumornecrosisfactor-α(TNF-α)conjugatefortherapeuticapplication,ScienceChinaLifeSciences,2013,56.1:51-58.
12.Dai, C.Y.,etal., LinkagewithcathepsinB-sensitivedipeptidepromotestheinvitroandinvivoanticanceractivityofPEGylatedtumornecrosisfactor-alpha(TNF-α)againstmurinefibrosarcoma, ScienceChinaLifeSci,2011,54(2):128–138.
13. Menkhorst,E.,etal.,VaginallyAdministeredPEGylatedLIFAntagonistBlockedEmbryoImplantationandEliminatedNonTargetEffectsonBoneinMice,PLoSONE, 2011,6(5)e19665.
14. Cai,Y.,etal.,Separationofexenatideanaloguemono-PEGylatedwith40kDApolyethyleneglycolbycationexchangechromatography,JournalofChromatographyA,2011,1218:39,P.6953-6960.
15. Wang,Y-J.,etal.,PEGylationmarkedlyenhancestheinvivopotencyofrecombinanthumannon-glycosylatederythropoietin:Acomparisonwithglycosylatederythropoietin,JournalofControlledRelease, 2010,145:3,p. 306-313.
16.Marcus,Y.,etal.,TurningLow-Molecular-WeightDrugsintoProlongedActingProdrugsbyReversIBLePegylation:AStudywithGentamicin, JournalofMedicinalChemistry,2008,51(14),4300-4305.
17.Wang,S.,etal.,Y-typepolyethyleneglycolmodifiedG-CSFandpreparationmethodandusethereof.PatentCN200780051378,2007.
18.Zhou,W.,etal.,Interferonalpha2amodifiedbypolyethyleneglycol,itssynthesisprocessandapplication.PatentCN200780050541,2007.
19.Zhou,W.,etal.,Interferonalpha2bmodifiedbypolyethyleneglycol,itssynthesisprocessandapplication.PatentCN200780050542,2007.
20.Zhou,W.,etal.,Double-strandedpolyethyleneglycolmodifiedgrowthhormone,preparationmethodandapplicationthereof.PatentCN200880009718,2008.
Foundedin2001byexpertsinPEGsynthesisandPEGylation,JenKemTechnologyspecializesexclusivelyinthedevelopmentandmanufacturingofhighqualitypolyethyleneglycol(PEG)productsandderivatives,andrelatedcustomsynthesisandPEGylationservices.JenKemTechnologyisISO9001andISO13485certified,andadherestoICHQ7AguidelinesforGMPmanufacture.TheproductionofJenKem®PEGsisback-integratedtoin-housepolymerizationfromethyleneoxide,enablingfaciletraceABIlityforregulatedcustomers.JenKemTechnologycaterstothePEGylationneedsofthepharmaceutical,biotechnology,medicaldeviceanddiagnostics,andemergingchemicalspecialtymarkets,fromlaboratoryscalethroughlargecommercialscale.
JenKem Technology由PEG合成和PEG化的专家成立于2001年,专门从事高纯度,低多分散性的聚乙二醇(PEG)衍生物,PEG共聚物,单分散PEG,定制PEG衍生物合成和PEG化服务。通过获得本体环氧乙烷以及内部所有聚合和衍生化的性能来支持特定的强度。
JenKem Technology从研发到GMP商业批量提供全球PEG衍生物,用于临床前,临床试验以及用于制药,生物技术,医疗设备和诊断的商业产品。













